intilaris biotech
services
intilaris services empower Biotech Management to succeed in today’s complex R&D.
intilaris LifeSciences who we are
A Swiss Life Science company delivering:
- Structured and Standardized Drug Development processes
- Alignment of the Biotech Research Results with the needs of Pharma Development Partners
- Compliance with applicable Quality and Regulatory requirements
- and a proven Critical Path to the earliest out-Licensing or regulatory approval.
The service portfolio aligns Pharma Needs
with Biotech Innovations.

Service for biotech companies and investors
Want to learn more about how we help Biotech clients?
Helping Biotech Clients succeed
Optimizing the Value of your Development Candidate
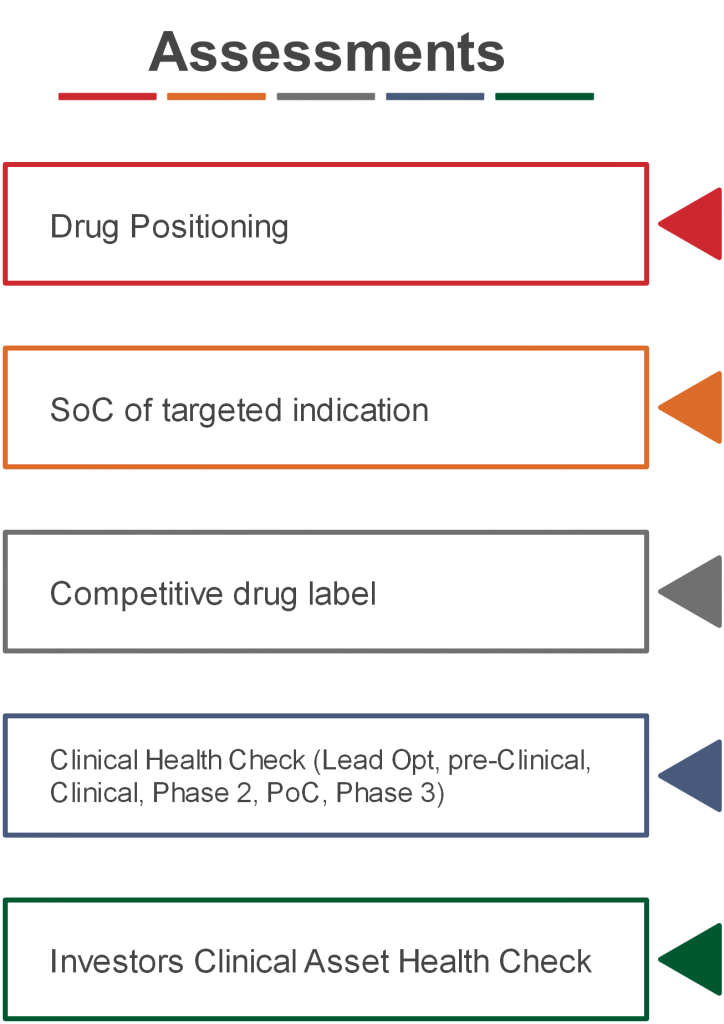
Drug Positioning
Positioning of the new drug on the future market. Align with commercialization demands and actively optimize your product profile.
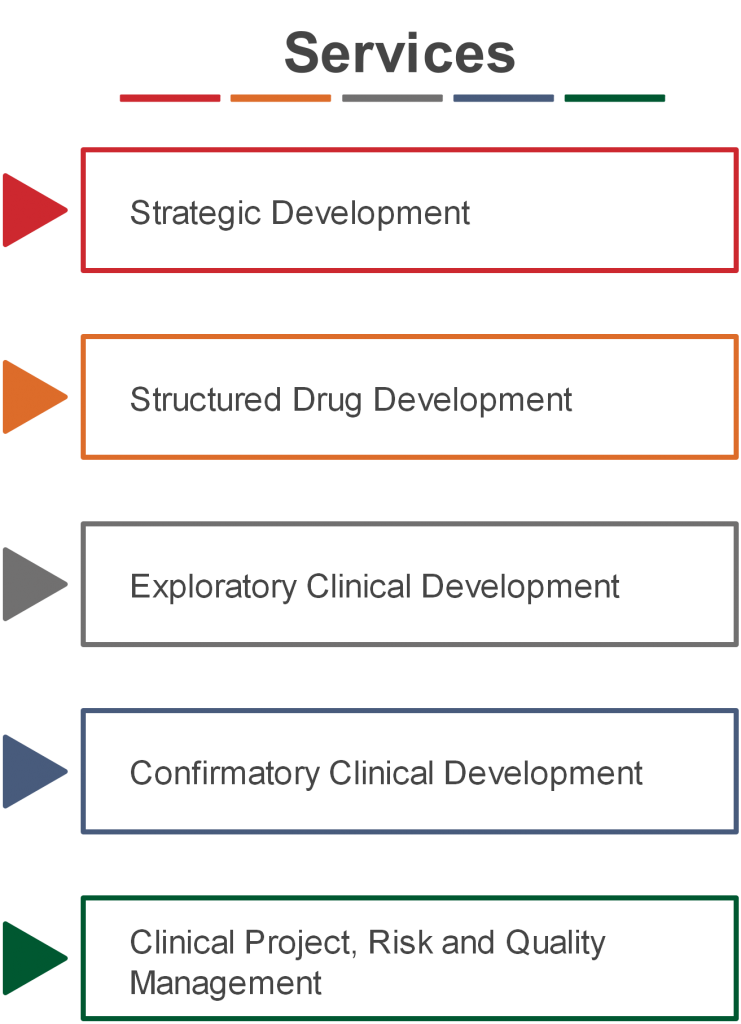
Strategic Development
Clinical trials are supposed to confirm your research assumptions, therefore a strategic and patient value focused Clinical Development Plan is essential for successful drug development.
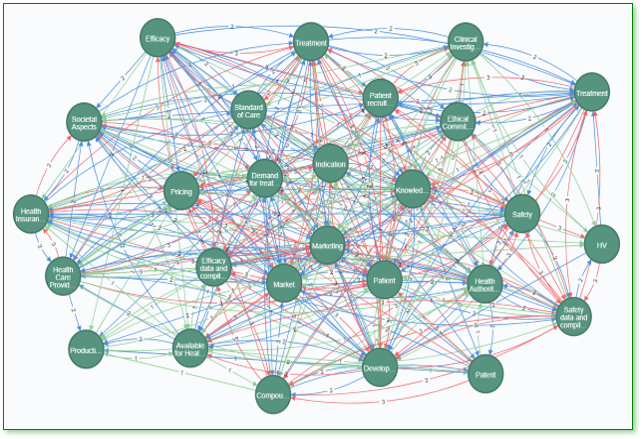
Assessments based on Systems Thinking
intilaris Biopharma New Product Assessment empowers Biotech Management to turn science into a commercially viable product in today’s complex R&D. This is achieved by benchmarking, optimizing and monitoring the Target Product Profile based on systems thinking.
Target Product Profile Modeling
The TPP (Target Product Profile) is critical for the strategic development planning to strengthen the position of the new product on the market.
We help you to define the product you would like to commercialize by integrating commercial aspects, such as medical need, added value, IP strength and others into Early Research & Development strategy.
The assessment reveals an optimal product profile and development options to maximize the product value.
This service “Biopharmaceutical New Product Assessment” is delivered in a structured workshop at major development milestones. The assessment requires minimal effort.
Drug Development outcome for the best product value
benchmark
optimize
monitor
Plan your development strategically and transparently
Structured Drug Development

Regulate
Our approach aligns the innovation with the regulatory needs.

Control
offers full control, transparency and a head start into a productive organization prepared for the future

Act
provides content based actionable information. Medical Standards build an effective synergy with Pharma

Improve
identifies deficiencies in current processes and study designs
Biotech products for your success
streamline drug development from Research to Proof-of-Concept (PoC)

Research
Establishing a clear and consistent vision for the future Product Development and early alignment with Pharma needs.
Delivered within a joint 5 day workshop with the Biotech Management team. The report
reveals deviations and determines effective tasks to remedy deficiencies.

Pre-Clinical
Strengthening the Product Claims and enriching with knowledge on Pharmacology, Toxicology and Formulation of the future product.
Creation of Target Product Profile (TPP) and Clinical Development Plan (CDP).
Delivered within a joint 5 day workshop with the Biotech Management team. The report
reveals deviations and determines effective tasks to remedy deficiencies.

Exploratory
Fast Track FiH (First in Human) to PoC (Proof-of-Concept) – Planning of Clinical Trials Phase 1 and 2a, supported by Structured Drug Development approach for optimal product positioning.
Delivered as a service through an assigned Clinical Development Project Manager, who works with your Team.

Confirmatory
Planning and design of Clinical Trials Phase 2b and 3 up to submission (NDA), supported by Structured Drug Development approach. Clinical TPP ensuring superiority of the new treatment compared to SoC. Trial design and protocol development in alignment with TransCelerate CPT industry protocol.
Delivered as a service through an assigned Clinical Development Project Manager, who works with your Team.
For Biotech Investors
Clinical Asset Health Check
Objective Clinical Asset Valuation
Places a rating on the likelihood of successfully completing the clinical development, receiving market authorization and generating revenue for the product(s).
Delivered as a workshop with Biotech Board and Management Team, followed by analysis (5-Day work package). Assessment Report recommends areas of rectification and its resulting impact on the rating.

Benefits for investors

Structured Risk Assessment of Biotech Drug Development and implemented risk mitigation

Transparency of Biotech Development phases

Streamlined Biotech Development for potential co - development licensing

Target Product Profile (TPP) Assessments to determine Biotech product differentiators and targeted peak - sales
Funding Support

Looking for
Funding?
Discuss the possibilities for your company with us.
Without any obligations.
Licensing Support
Need support for
Licensing?
Discuss the possibilities for your company with us.
Without any obligations.
We offer:
- Assessment of assets which you seek to license (or seek to obtain a license)
- Competitive intelligence on market deal structure and terms
- Preparation of materials to engage with the market
- Selection and interaction with prospective partners and deal advisory/negotiation services
Latest news
Structured Drug Development
What our clients say


Let's talk about your next project
Mitigating Risk & Creating Value by Aligning Drug Development & Commercialization