Who we are
A Swiss Life Science company delivering
- effective Clinical Development planning, Study Designs and Protocol support
- Compliance with Quality and Regulatoryrequirements
- a proven Critical Path to the earliest out-licensing or regulatory approval
- and a Clinical Asset Health Check for Investors to ensure funding


Torsten Friedel
Director Consulting
Process excellence and focused customer solutions 26+ years pharma industry and consulting

Dr. Djenan Ganic
Director Business Solutions
Business Solutions and Development 19+ years pharma industry expertise and consulting
Learn more about
our work methods
intilaris is a Swiss life science consultancy
providing Drug Development support for innovative Biotechs. We help defining the product you would like to commercialize (TPP) by integrating commercial aspects into Early Research & Development strategy.
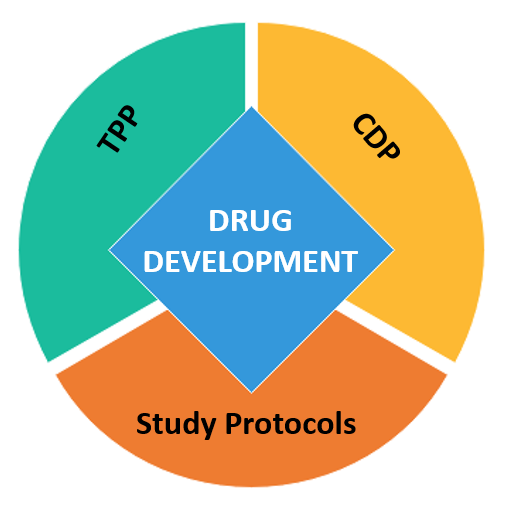
Biotech teams are supported by intilaris specialists in the strategic planning process. The Clinical Development Plan (CDP) in alignment with the TPP, captures essential trial designs on the critical path to registration.
intilaris helps Biotechs to design Clinical Trials and draft Study Protocols focusing on patient safety and credibility of the trial results. Biotechs benefit from best practice Clinical Trial planning and streamlined operational execution.

